Introduction
Many pharmaceutical and medical device companies encounter the need to establish cleanrooms at one stage or another.
When it comes to startups, they usually do not have the knowledge and resources to set up such a project. And they turn to professional suppliers, but for some reason, the results are not always is satisfied. Why?
Based on our experience of dozens of projects (cleanroom establishment and remediation), no matter if
- Poor design
- Poor execution
- Inappropriate materials usage
- Lack of budget
- Lack of professionalism of such or other factors in the project
ultimately supervision in the planning and execution stages by a professional who understands the regulatory requirements, the manufacturing process, and the engineering requirements is the key to get a valid manufacturing facility capable of supporting good manufacturing.
Cleanroom Design
A cleanroom is a room or space where particulates (viable and no viable) are controlled and monitored.
The construction level, temperature, relative humidity, airflow patterns, air exchanges, ingress and egress of personnel and materials, and pressure regime are controlled to reduce the risks.
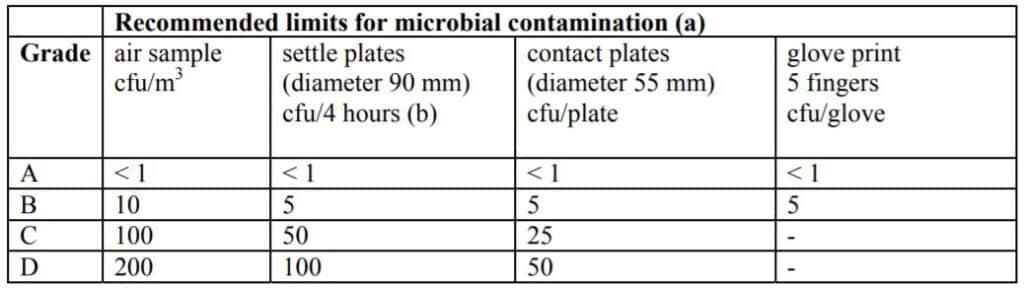
In the Life Science industry, the level of viable particles (microorganisms) poses a risk of infection to the patient following the product contamination should also be controlled.
The cleanrooms classifications are common in the pharma, medical device and medical cannabis industries, where the product’s cleanliness level is critical.
(The Cleanrooms are common in other sectors, such as electronics and more, but in this article, we will refer to cleanrooms for the Life Science industry).
This article covers multiple topics, such as:
- Cleanroom Classifications
- Emphasis on planning and building a cleanroom
- HVAC (Heating, Ventilation and Air Conditioning) systems design
- Airflow pattern
- Air Supply Rate
- Filter Efficiency and Space Layout
- Air Pressure Control in Cleanrooms
- Temperature and Relative Humidity
- HVAC system design cost-effective consideration
Cleanroom Classification
There are two main regulations for classifying the air’s cleanliness in cleanrooms.
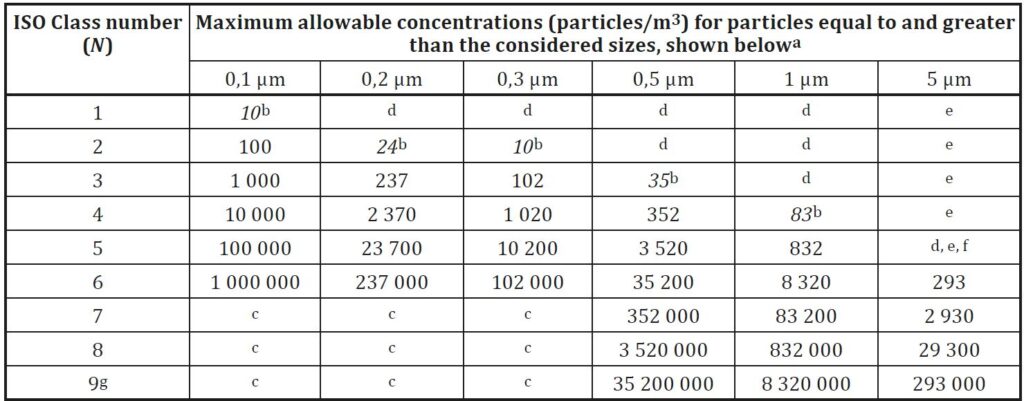
One approach is based on ISO standards for cleanrooms (ISO-14644) and defines classifications from the ISO-1 level (the most stringent) to the ISO-8 level.
For Medical device, Biotechnology, Pharmaceutical industry, the classification of the cleanrooms from ISO-5 to ISO-8. This method is more common in the US market.
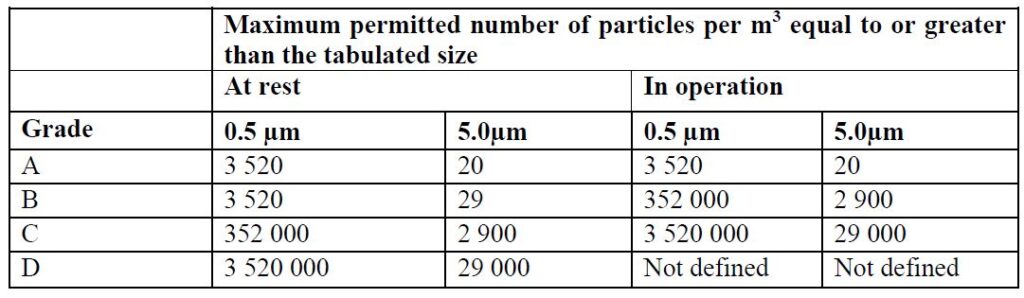
The approach used in the EU-GMP market, based on Annex 1, is from Grade A (the most stringent) to Grade D.
The above two methods are based on defining the number of particles allowed per unit of air volume in the room space. The particle size is measured in micron units.
When working with both standards, there is a reference to the room’s condition, whether the room is at work (In-Operation) or at-rest (At-rest).
Accordingly, there is a requirement for the level of cleanliness of the air from particles in any situation.
Should be mentioned that Controlled Not Classified (CNC) areas shoul also be considered and defined.
Emphasis on planning and building a cleanroom
First of all, it is essential to emphasize that a cleanroom is not a stand-alone room but is usually a complex consisting of cleanrooms in different classifications with corridors and air-locks connecting them.
The entire complex is supported by an air conditioning treating system (Heating, ventilation, and air conditioning- HVAC).
The cleanroom requires a high construction finish quality and should be built with walls and ceilings sealed and wholly separated from outdoor air and have a smooth and easy-to-clean interior finish.
Cleanrooms are kept under positive or negative pressure, so sealing the cleanroom is critical to maintaining defined pressure.
The cleanroom’s interior surfaces (ceilings, walls, floors, furniture, windows, and doors) must be resistant to detergents and cleanable surfaces.
Cleanrooms can be built using conventional construction methods to fit the room classification, so the interior finish must be designed.
ISO-8 cleanrooms may require only epoxy-painted walls; however, this will depend on the particle levels created by the people and the processes in the room. Ideally, all interior walls should be made of rigid metal or plastic panels attached to the wall frame.
An alternative to conventional construction techniques is the use of modular wall systems. Smooth surfaces and airtight connections reduce air leakage and provide excellent environmental performance.
HVAC (Heating, Ventilation and Air Conditioning) systems design
HVAC systems’ design in cleanrooms is different from that of commercial buildings.
Typically, the most common HVAC parameters for classified spaces include:
- airborne particles (air cleanliness), including viable particles that accompany non-viable particles
- the temperature of the air
- the relative humidity of the air
HVAC can control only airborne conditions and cannot remove deposited (surface) contaminants. Airborne contaminants can settle and create surface contamination. Surface contamination can also be disturbed to create airborne contamination.
The cleanroom temperature and relative humidity might influence the comfort of operators who are present and, therefore, the number of particles (viable and not-viable) shed by them.
A high degree of protective gowning may minimize contamination from operators, but cooler temperature and lower relative humidity may be needed to keep operators comfortable with more gowning.
Other HVAC variables, such as room relative pressure, HEPA filter integrity, airflow patterns, and airflow volume, can affect one or more of the above parameters.
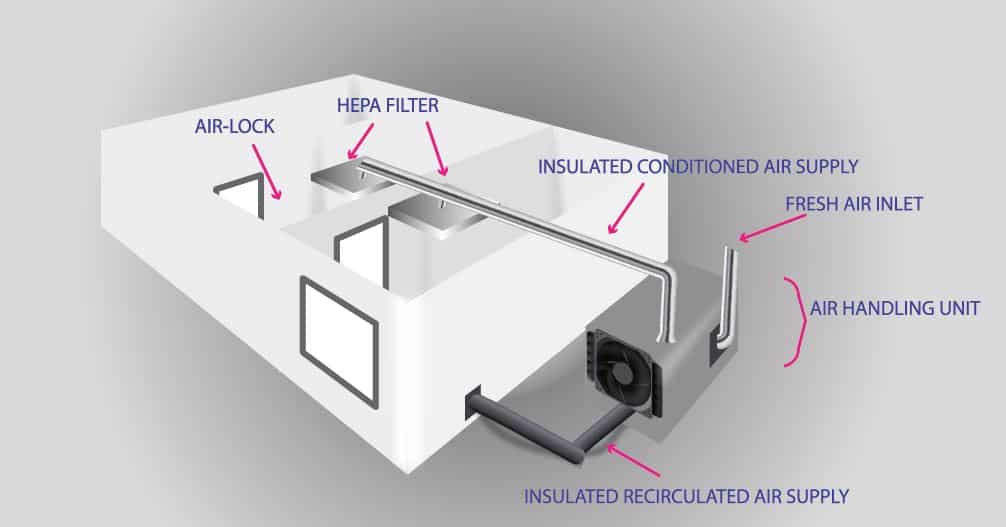
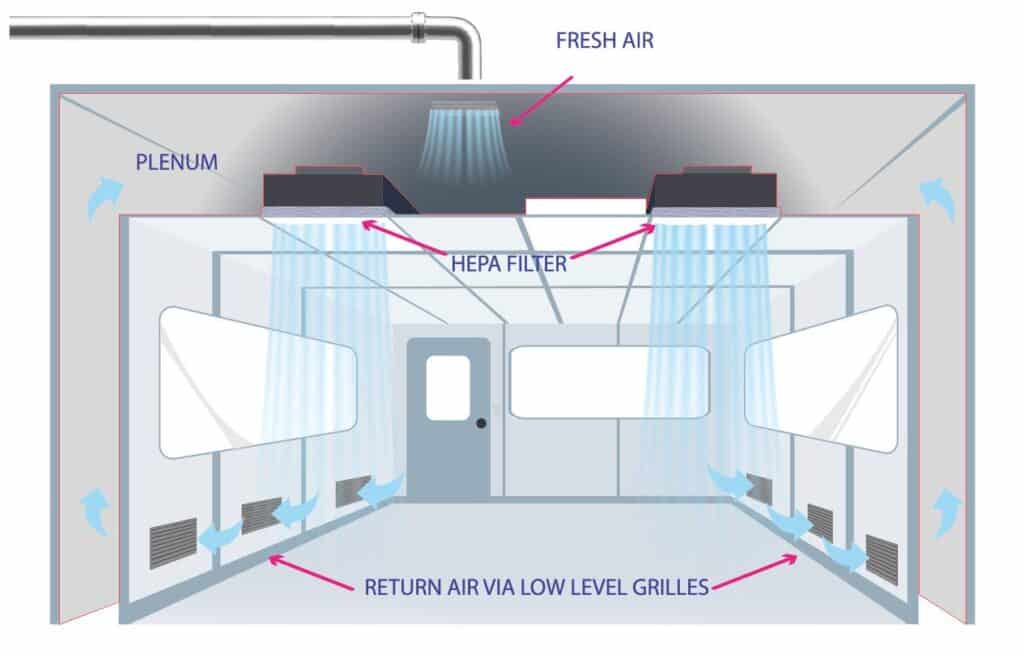
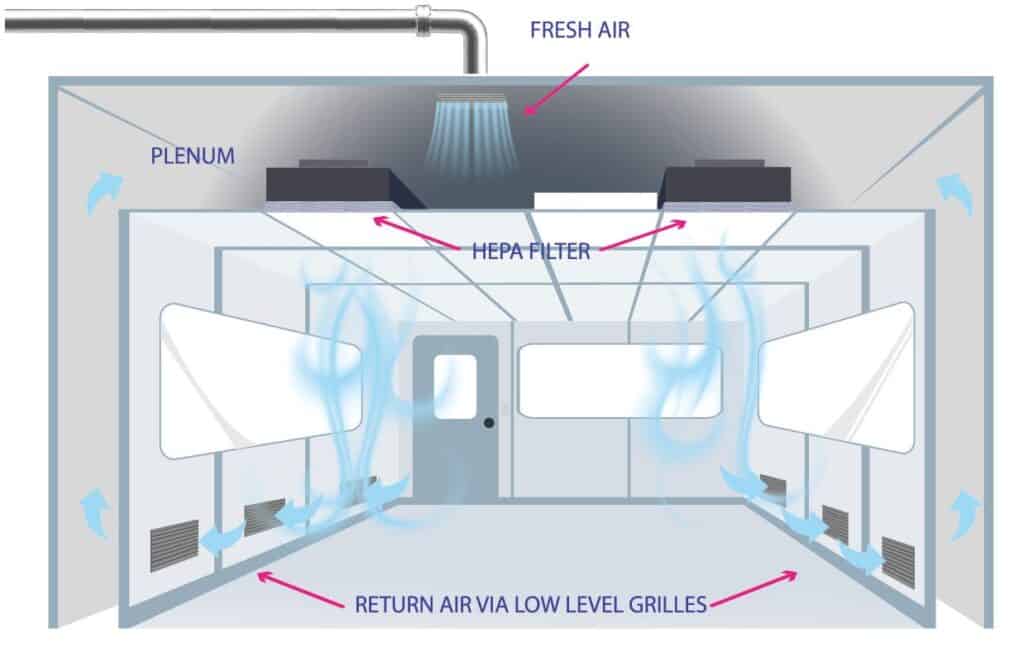
In the schematic drawing: Air enters through a unit for air treatment, where it undergoes initial filtration as well as temperature and humidity treatment.
This air passes through air conditioning ducts and enters into a cleanroom through HEPA filters. Air returns from rooms through a grill inside the room to the plenum and air conditioning ducts lead the “dirty” air out or return to the rooms through an air treatment unit.
Airflow pattern
There are two types of airflow patterns for cleanroom design:
Type 1: Unidirectional (Laminar flow)
Airflow pattern in which essentially the entire body of air within a confined area moves with uniform velocity in a single direction with generally parallel air streams (Grade A/ ISO 5).
Type 2: Non-unidirectional flow (Turbulent flow)
Airflow is non-unidirectional by having a varying velocity, multiple pass circulation, or nonparallel flow direction (Grade B, C, D/ ISO 7,8)
Cleanroom design practice recognizes that environmental conditions equal to Grade 7 can be achieved, in operation, by turbulent airflow dilution.
However, higher standards in operation, such as Grade 5, are achieved by a displacement system.
The inherent very high air change rates (hundreds per hour), high capital costs, and operating costs associated with “electronic” industry-type displacement (unidirectional flow) cleanrooms are not common for most pharmaceutical aseptic cleanrooms.
However, dilution design (turbulent flow) also places high demands on HVAC system design. It must take room layout and operations into account, as well as providing adequate local unidirectional airflow to protect critical areas.
It is particularly important to identify low air movement areas that may give rise to pockets of higher particle concentrations (for example, in-room corners where the impact of particles is low). Critical process operations should not
be situated in these areas.
Air Supply Rate
Conventional air conditioning systems in the home or office require about 4 to 8 air changes per hour. In comparison, the cleanroom can require between 20-40 air changes per hour and even more, depending on the classification.
Since this is a cleanroom complex, the air conditioning system must be designed with sufficient flexibility to provide the optimal level of cleanliness in the various rooms.
Annex 1 of the EU cGMP covers various aspects of sterile product manufacture, including contamination control.
It suggests that manufacturing cleanrooms will quickly recover from the generation of high concentrations of airborne particle contamination and should ‘clean up’ in 15 to 20 minutes (guidance value) after completion of operations, to the particle limit stated for the ‘at rest’ state.
This applies to cleanrooms designated Grade B and Grade C, which have non-unidirectional airflow but not Grade A cleanrooms with unidirectional airflow, or Grade D with no specified ‘operational’ particle concentration.
Achieving this ‘clean up’ time is considered to demonstrate that the cleanroom has ventilation effectiveness that cannot be guaranteed by a simple air supply rate specification, which provides the required airborne contamination in the steady-state condition.
Filtering Efficiency and Space Layout
Although a HEPA filter can capture over 99.97% of particles (both larger and smaller than 0.3 microns), it is Good Engineering Practice to provide the system with prefilters to capture larger particles representing most of the mass, therefore extending the life of the final HEPA filter.
For this reason, some configurations employ double HEPA filtration with the primary filter fitted in the air handling unit and the secondary filter (terminal filter) located within the classified zones.
Depending on the system types, a pressurized plenum or ductwork is connected to HEPA filters in the ceiling.
The ceiling coverage by HEPA filters varies with design requirements. Often, the ceiling coverage by HEPA filters can be anywhere between 25% up to 100% (when the grade is higher, the coverage by HEPA filters in the room increase).
With lower ceiling coverage, the airflow’s face velocity in the filter would tend to be higher, resulting in more fan power demand. Careful considerations should be given when selecting filter efficiency and space layout.
Air Pressure Control in Cleanrooms
The guiding principle is to maintain the pressure differential across the activity complex and prevent microbiological contamination or cross-contamination of the product.
In a clean complex with several rooms, the pressure in sensitive areas should be kept high from the adjacent rooms to ensure that pollutants do not move to the most sensitive areas.
Particles entering a room from an adjoining room of lower air classification may be controlled by room pressurization.
Air-locks are the preferred method of preserving Differential Pressure (DP) between different classification rooms. A positive room DP helps exclude external contaminants, reducing infiltration from more contaminated spaces.
Where a doorway separates cleanrooms of different air quality classifications, an air-lock should be used to prevent doors opening simultaneously, thus maintaining DP in the joined cleanrooms.
Airborne particle levels in an air-lock should meet the same at-rest levels as the highest quality room served by the air-lock.
If there is no air-lock, DP across the door essentially goes to zero when the room between air classes is opened, even though there should be noticeable outward airflow. Limits and alerts should be placed when a door can remain open.
Temperature and Relative Humidity
An air handling unit regulates moisture and temperature with support systems’ help.
Temperature and relative humidity for rooms housing critical operations should be monitored to assure that operator comfort and prevent microbiological growth.
Occasionally a product may have a stringent temperature and relative humidity requirement.
HVAC system design cost-effective consideration
When designing new or renovated facilities, the goal is to provide a facility design that makes the best use of available technology to assure product quality while remaining cost-effective.
This involves both a risk assessment approach and a broad understanding of available technologies and increasing regulatory requirements.
The result is that a facility designed to meet the intent of its use by procedural means is a very different facility from one designed incorporating spatial means and different again from a facility designed for time-based approaches.
The life cycle cost of facilities and not just the initial cost should be considered. A higher initial cost using better materials may mean less operating and maintenance costs and, therefore, lower life cycle costs.
Final Thoughts
Cleanrooms form the core for manufacturing companies in the Life science industry. The cleanroom creates an environment that directly impacts manufacturing processes and the consistent production of quality products.
Without a suitable cleanroom to drive your system, production will be distracted, delaying delivery to customers. Cleanrooms have many potential pitfalls, and supervision by an engineer with extensive cleanroom experience is a must.
As an expert in this field, we will guide you and manage this complicated project to align all stakeholders with your own needs and regulatory requirements.
If you have any questions, please feel free to contact our experts, who’ll be more than happy to discuss your inquiry in further detail.
RS-NESS works with various companies to construct production facilities and provide a comprehensive solution for these complex projects.
We accompany the customer from the initial stages – planning in principle, obtaining facility approval from regulatory bodies and perform validations for facility / equipment / systems / processes while accompanying and supervising the entire project on behalf of the customer.
If you have any questions, or if you need professional support, please contact us.



















