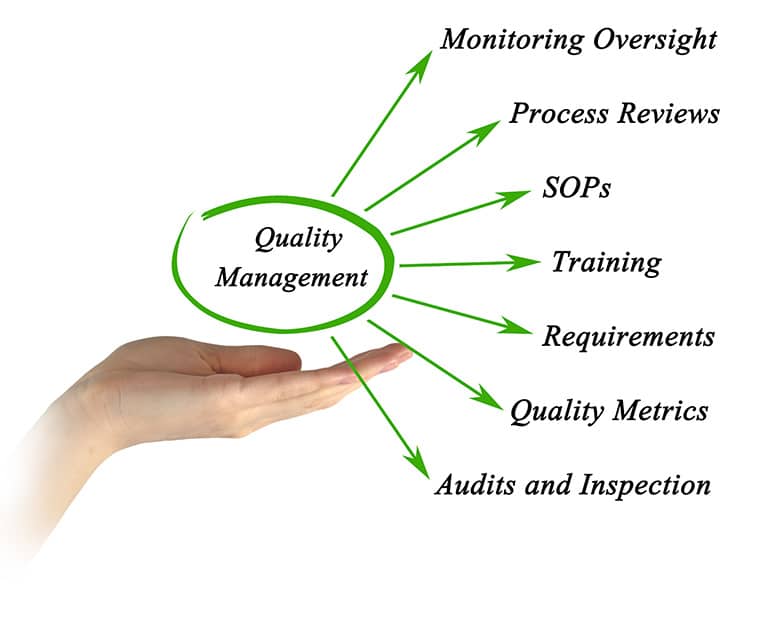
Quality assurance is one of the most important areas in any project and ongoing activity. In R&D, QA is the last frontier before production and live operation, and should be done with full attention to details along with viewing the full picture, and as a flawless and precise process. We at R.S NESS Group take into consideration the QA during the engineering, planning and characterization stages, by creating the proper documentation, writing the test cases and making sure that all user scenarios are covered, and all system tests are performed properly. We see the full cycle of a product from the start, and our vast experience, out of the box thinking and overall view of the system in an operational environment enables us to plan and execute QA activities in the best and optimal manner.
As part of our QA approach, we work together with the R&D teams, engineers and product managers, to reach the mutual goal of highest quality products and outputs, while ensuring that your requirements are fully met.
Management Systems according to ISO9001: 2015, ISO13485: 2016, 21CFR820, Q10