You have a medical device product that has been on the market for a short period of time with FDA approval. You know the science behind it is great, and sales have been doing well. You’ve managed to ship the product all around the world, but suddenly you start to receive customer complaints…the product has had instances of reaching the customer damaged and sometimes even partly open. Now you start to get worried and the following concerns start to flow in:
- Are we going to need to recall products?
- How will this ruin the company’s reputation?
- How much money are we going to lose?
- Can we trust the packaging validation we previously conducted? If not, what should we have done?
You’ve come to the right place. At RS NESS we have extensive knowledge in the process of Packaging Validation and can provide you with the support, information and tools you need every step of the way.
Let’s start with the basics
First and foremost, packaging validation is a regulatory requirement for Class II and III medical devices. However, apart from the regulatory approval perspective, there is a wide range of benefits to conducting packaging validation onto your package. Let’s look at a few of them.
Packaging validation ensures that when your product reaches the customer it is:
- Preserved and protected
- Safe and fit to use and/or consume
- Identifiable and informative
- Sterile/microbe-protected.
These are not necessary requirements for all products and they will differ depending on whether your product is a medical device or a pharmaceutical product. At the end of the day, what matters most is the customer’s safety, satisfaction, benefit and the company’s reputation.
In terms of where you start – packaging validation is a process which consists of a number of steps. It all begins with the following:
Product Information Gathering
Product information gathering is a critical first step – it will decide all future stages for packaging validation. It is here where we list and understand every detail of our medical device product’s packaging and shipping.
The details we want to collect in this stage will look like this:
- What equipment/machinery is required to package our product? What processes does it go through?
- Is our product sterile/aseptic?
- How is our product packaged? What packaging material is directly touching our product (primary packaging)?
- What packs our primary packaging i.e. what makes up our Secondary and Tertiary Packaging, including logistical packaging?
- Are all the above packaging materials compatible? Can they react with one another?
- How do we get our product from A to B? What does its supply chain process look like? How many steps does it go through?
In addition to this, the following has to be final:
- Standard Operating Procedures (SOPs): Operation, Maintenance and Cleaning of machinery/equipment used to package product. This tells us that your packaging process is under control.
- Calibration and Maintenance Program
- Operator Training for equipment/machinery.
Product Damage Impact Assessment
Once you’ve understood your product and how it is shipped and the processes involved to make the packaging, we now risk assess. We start to paint the picture of potentials – if things were to go wrong during production or during shipping etc – what would the impact be?
It is here that we understand what is important to us, our risks at every stage of the process, and it is the opportunity to find mitigations to deal with those risks.
It is important to point out that validation is always risk-based – therefore it is in your interest to find mitigations to reduce your risks as much as possible. This may save you time and money, in addition to making your process/product safer. It might be a great opportunity here to also look at the recent customer complaints and add risks which may have been previously missed.
How do we go about this?
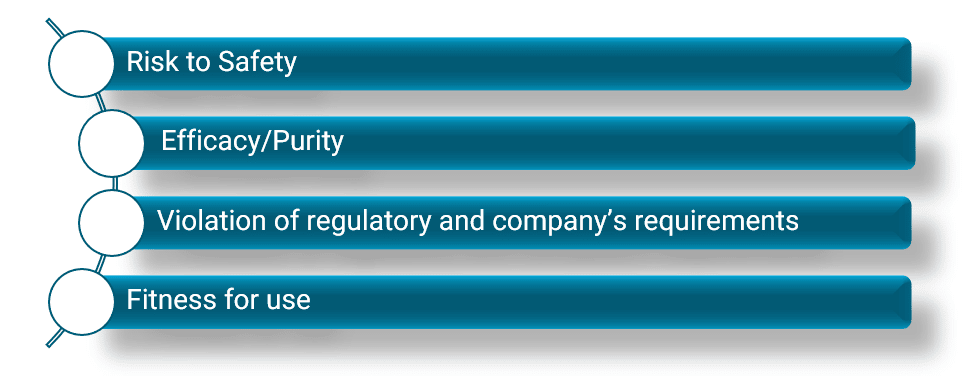
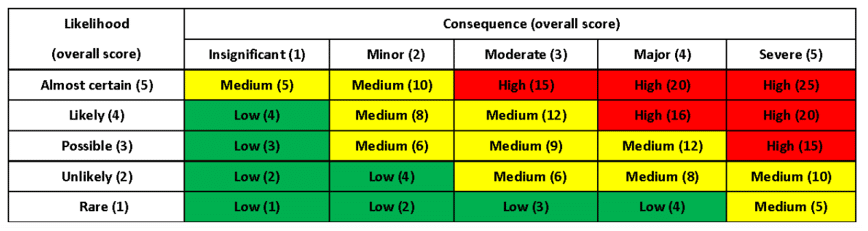
We create a risk matrix to understand the likelihood vs. consequence of failures in the following attributes to obtain a risk score:
The risk matrix generally looks like this, but can be adjusted to fit your company’s policies:
A team of people from different departments discuss and decide how each risk is rated. You choose the relevant likelihood and consequence, and then find its appropriate place on the risk matrix. You should implement mitigation strategies here to move your risks from the red zone to the green zone, which is desired and safer. In some cases, you might be able to do that by implementing engineering controls. It is vital to ensure that all of this is documented.
Testing Selectio
We’ve reached the stage where we understand our product well, and we now also understand the risks involved in the packing process and the packaging itself. With this information, we can now make an informed decision (together with the relevant guidelines) about the tests required to make our packing process and the packaging validated. All testing is outlined in a Master Validation Plan, and the details are to be set out in relevant protocols.
Testing your packing process and equipment
With the help of the Installation Qualification (IQ), Operational Qualification (OQ) and Performance Qualification (PQ) protocols, you are able to validate your packing process. The sample size to be produced and tested at this stage would depend on your risk assessment and statistics. The samples then undergo the below steps.
Testing your packaging
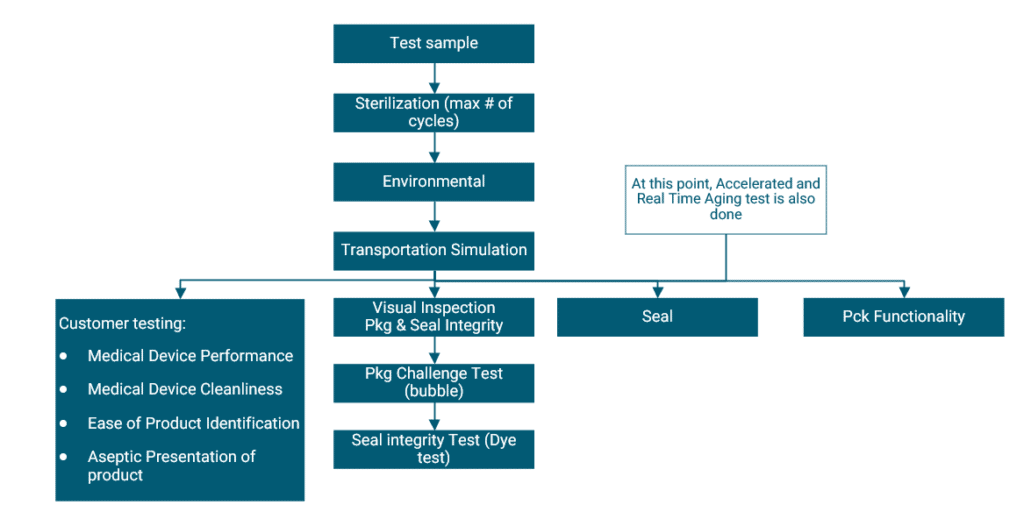
According to ISO11607, testing of your packaging is conducted under 3 conditions. The Baseline conditions form the framework, and the other 2 add an accelerated or real-time ageing step. This allows you to understand the impact of time on your package (and product within it) under these tests. The test flow chart below provides a clear picture of the tests involved in a sterile medical device product.
Environmental conditioning is conducted for products that are sensitive to changes in temperature and humidity. It exposes your package to a simulated environment for a specific amount of time according to ISTA 2A:2011, putting your package in “worst-case” conditions. Transportation simulation testing is then conducted – here the shipping and transportation process of your package is simulated according to ASTM D4169:2016. There are a number of different cycles to choose from – and the one that most resembles what your product will go through during shipping is to be chosen.
Following these simulations, your package will undergo a number of inspections and tests to challenge its integrity and functionality.
Guidelines
When it comes to Validation, guidelines are your best friend – they must be understood and complied with from beginning to end. For our purposes, useful resources would be the following:
- Packaging Validation: ISO11607-1 and -2
- Risk assessment:
- For Medical Devices: See ISO 13485:2016
- For Pharmaceuticals: See ICH guideline Q9
- ISTA 2A:2011
- ASTM D4169:2016
Final tips
- Don’t be afraid to get into the nitty-gritty details of your product and processes
- Ensure that all risks are understood
- Be consumer centred
- Do the right thing
- Always comply with regulations
RS NESS is a highly experienced service provider in the Medical industry. RS-Ness can assist you with Packaging and Shipping valiation, to help you create an appropriate project plan, and support the transition throughout the Regulatory, Clinical, QA, Validation, and Engineering processes.
We differentiate ourselves by being quality-oriented and by our technical expertise that comes with a hands-on experience and approach, ensuring that our clients receive the most effective and professional service.
Our clients range from small Start-Ups to international companies.
If you have any questions, or if you need professional support, please contact us.
About the author

Linor Skutelsky
QA Engineer and Project manager
Highly experienced in quality assurance in the Medical Device industry. In the past four years, Linor participated in many projects.
Linor earned her Bachelor of Science degree in Biotechnology Engineering from Ben Gurion University, Be’er Sheva, Israel, and her Masters of Science degree in Environmental Engineering from the school of Mechanical Engineering, Tel Aviv University, Tel Aviv, Israel.