Preparing your QMS for the EU MDR

RS Ness supports Life Science companies in QA, Regulatory Affairs, Clinical Affairs, Engineering, Validation and Project Management.
Contact us to support your MDR Transition.
How to establish DHR

RS NESS supports the Life Science in such field as: Quality Assurance (QA), Regulatory Affairs (RA), Clinical Affairs (CA), Engineering, Validation and Project Management (PM). We provide service to the companies at different lifecycle stages incorporating end-to-end project activities while adhering to the regulatory requirements. Knowledge, professionalism, and dedication lead our highly qualified team to your success.
If you have any questions, or if you need professional support, please contact us.
Medical Device Roadmap from Idea to Market
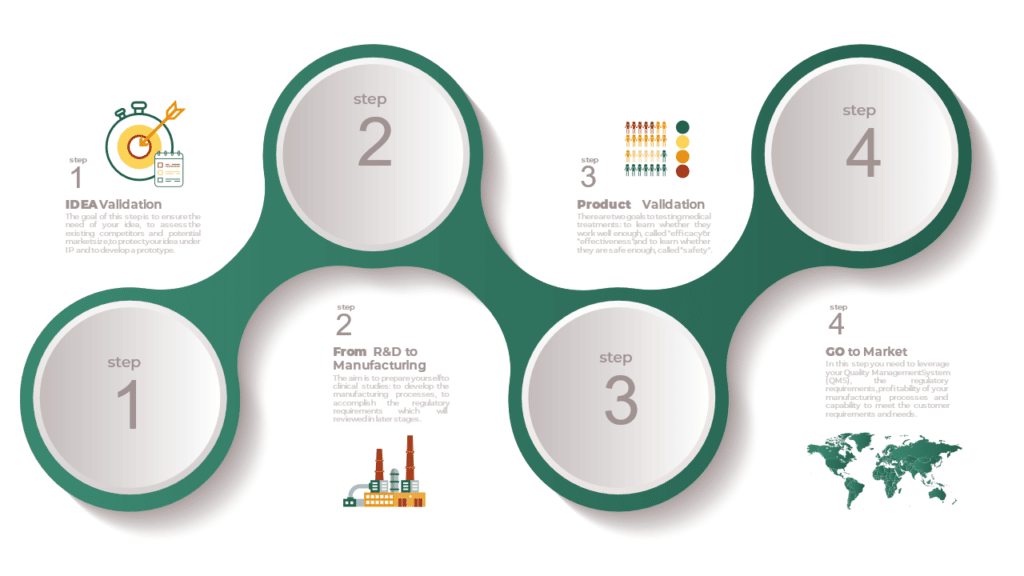
Commercializing medical technology is challenging, often requiring 10 – 20 years just to bring new ideas to market. There is an essential need for better understanding of the overall process to the market. Challenges arising from time delays, misalignment with regulatory requirements and resource constraints potentially amount to substantial losses in value, for both companies […]
Your Packaging Validation Guideline

RS NESS is a highly experienced service provider in the Medical industry. RS Ness can assist you with Packaging and Shipping validation, to help you create an appropriate project plan, and support the transition throughout the Regulatory, Clinical, QA, Validation, and Engineering processes.
We differentiate ourselves by being quality-oriented and by our technical expertise that comes with a hands-on experience and approach, ensuring that our clients receive the most effective and professional service.
Our clients range from small Start-Ups to international companies.
If you have any questions, or if you need professional support, please contact us.
The Nanosono journey- from idea to ISO13485 Certification
RS NESS supports Medical device companies in rapid scale-up and meeting GMP compliance requirements; proving the experience you need when you need it.
If you are planning to get ISO13485 certification, please contact us for support.
Validation of Medical Device manufacturing line for getting the European CE mark certification.

RS NESS is a highly experienced service provider in the Medical industry.
RS-Ness can assist you with a gap assessment to help you create an appropriate project plan, and support the transition throughout the Regulatory, Clinical, QA, Validation, and Engineering processes.
We differentiate ourselves by being quality-oriented and by our technical expertise that comes with a hands-on experience and approach, ensuring that our clients receive the most effective and professional service.
Our clients range from small Start-Ups to international companies.
If you have any questions, or if you need professional support, please contact us.














