Medical Device Roadmap from Idea to Market
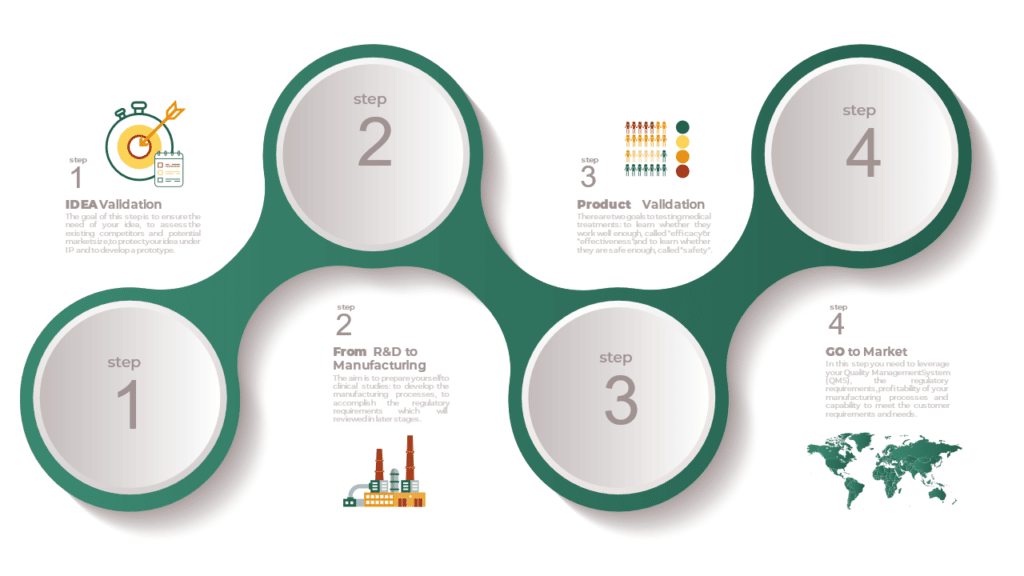
Commercializing medical technology is challenging, often requiring 10 – 20 years just to bring new ideas to market. There is an essential need for better understanding of the overall process to the market. Challenges arising from time delays, misalignment with regulatory requirements and resource constraints potentially amount to substantial losses in value, for both companies […]
Your Packaging Validation Guideline

RS NESS is a highly experienced service provider in the Medical industry. RS Ness can assist you with Packaging and Shipping validation, to help you create an appropriate project plan, and support the transition throughout the Regulatory, Clinical, QA, Validation, and Engineering processes.
We differentiate ourselves by being quality-oriented and by our technical expertise that comes with a hands-on experience and approach, ensuring that our clients receive the most effective and professional service.
Our clients range from small Start-Ups to international companies.
If you have any questions, or if you need professional support, please contact us.
The Nanosono journey- from idea to ISO13485 Certification
RS NESS supports Medical device companies in rapid scale-up and meeting GMP compliance requirements; proving the experience you need when you need it.
If you are planning to get ISO13485 certification, please contact us for support.
Validation of Medical Device manufacturing line for getting the European CE mark certification.

RS NESS is a highly experienced service provider in the Medical industry.
RS-Ness can assist you with a gap assessment to help you create an appropriate project plan, and support the transition throughout the Regulatory, Clinical, QA, Validation, and Engineering processes.
We differentiate ourselves by being quality-oriented and by our technical expertise that comes with a hands-on experience and approach, ensuring that our clients receive the most effective and professional service.
Our clients range from small Start-Ups to international companies.
If you have any questions, or if you need professional support, please contact us.
Randy Hoffman

General Manager Of RS Ness America
Supplier evaluation and approval in 5 steps.

RS-NESS supports Life Science companies in rapid scale-up and meeting GMP compliance requirements; proving the experience you need when you need it.
If you are planning a Supplier Evaluation and Approval process, contact us to discuss how we can support your goals.
Preparing your Quality Management System for the MDR: QA Best Practices

RS-Ness supports Life Science companies in QA, Regulatory Affairs, Clinical affairs, Validation and Project Management.
We will assist you with an MDR gap assessment to help you create an appropriate project plan, and support the transition throughout the Regulatory, Clinical, QA, Validation, and Engineering processes.
Our clients range from small Start-Ups to international companies.
If you have any questions, or if you need professional support, please contact us.
Moti Loitner

Engineering Director
If you have 2 months to establish an EU-GMP compliant production line for Medical Cannabis, what should you do?

RS NESS supports Life Science companies in rapid scale-up and meeting GMP compliance requirements; proving the experience you need, when you need it.
If you are planning your production validation, contact us to discuss how we can support your goals
Liat Aharon Pollak

Regulatory Affairs Director
Aline Goldstein

QA Sector Manager
Process Validation: Pharma vs. Medical Device

RS-NESS provides an umbrella of services to the Life Science industry in the areas of validation, quality assurance, regulatory affairs, clinical affairs, project management, and engineering. This approach enables a “one-stop-shop”, incorporating end-to-end project activities while adhering to the regulatory requirements. Knowledge, professionalism, and dedication lead our highly qualified team to your success.
MDSAP In 60 Sec

For those who are not familiar with the Medical Device Single Audit Program (MDSAP), it is a harmonized approach for auditing and monitoring medical device manufacturers quality management system on an international scale. MDSAP program allows a single regulatory audit of a medical device manufacturer’s quality management system to satisfy the needs of participating regulatory […]












