Ensuring Business Continuity in Life Sciences

A Critical Pillar for Quality and Compliance In the world of life sciences—whether medical devices, pharmaceuticals, or biotechnology—business continuity is far more than a strategic buzzword. It is an operational imperative that directly impacts patient safety, regulatory compliance, and long-term business viability. At RS NESS Group, we support companies across the life sciences spectrum in […]
BD case study

How medtech giant BD uses R.S NESS Group to meet global regulatory standards at infusion pump plant Israel-based Caesarea Medical Electronics, a global infusion pump systems manufacturer, was acquired by global medical technology giant BD (Becton, Dickinson and Company) (NYSE: BDX) in 2017. The move expanded BD’s infusion portfolio to include ambulatory, home and specialty […]
Poka Yoke and Six Sigma

Poka Yoke or Mistake Proofing is a technique for eliminating defects by making it impossible to create them in the process. It is often considered the best approach to process control. It works on the principle – “It is good to do it right the first time. It is even better to make it impossible […]
Computer System Validation

INTRODUCTION No matter what core services your company provides, if computer systems and software products are involved, efficiency will always remain one of your top priorities. This is not only due to the fact that efficiency ensures your customers receive the best service but also because inefficiencies and faults in computer systems can potentially result […]
Risk Management in Medical Device

Risk Management in Medical Device The EN ISO 14971:2019 standard specifies a process through which we medical device manufacturer can identify hazards associated with the medical device, estimate and evaluate the risks associated with these hazards, control these risks, and monitor the effectiveness of the controls throughout the life cycle of the medical device. In […]
What are Clinical Trials?

Clinical trials are any type of human research that is designed to: Detect or validate clinical, pharmacological and / or pharmacodynamic effects of a research product Identify any adverse reactions to the research product, Study product absorption, distribution, metabolism and excretion Determine or ensure the product’s safety and / or effectiveness. The terms clinical trial […]
Deviation Management Process
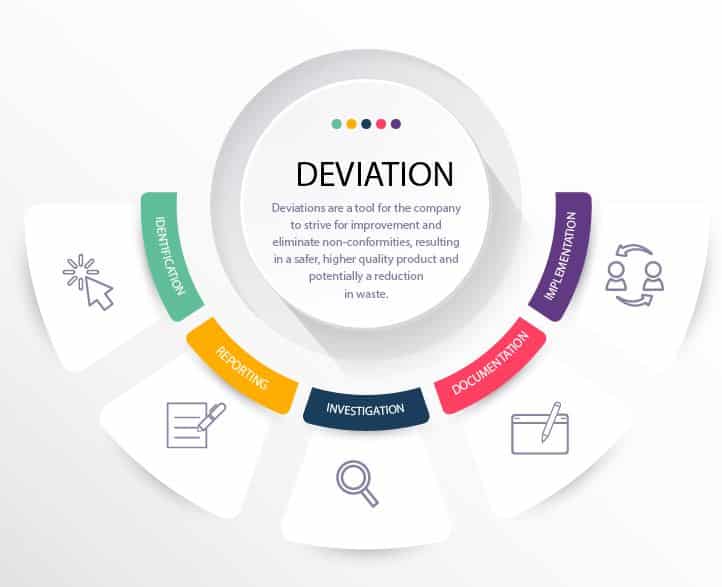
RS NESS is a highly experienced service provider in the Medical Device and Pharmaceutical industries.
We can assist you with CAPA and Deviation Management in helping you to create appropriate policies and process, as well as to optimize deviation handling.
Purchased Part Approval Process (PPAP)- the next step in supplier quality
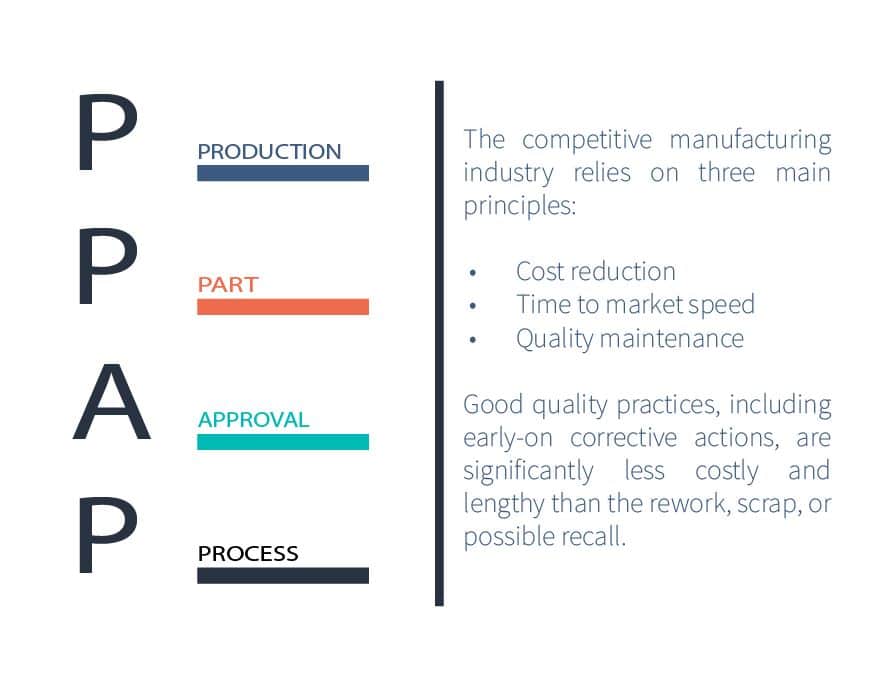
RS NESS is a highly experienced service provider in the Medical industry.
We can assist you with a PPAP process implementation to help you create an appropriate and suitable process for your needs and improve your supplier quality.
Our expertise is in the field of Regulatory Affairs, Clinical Affairs, QA, Validation, and Engineering processes.
IVD Classification Under IVDR
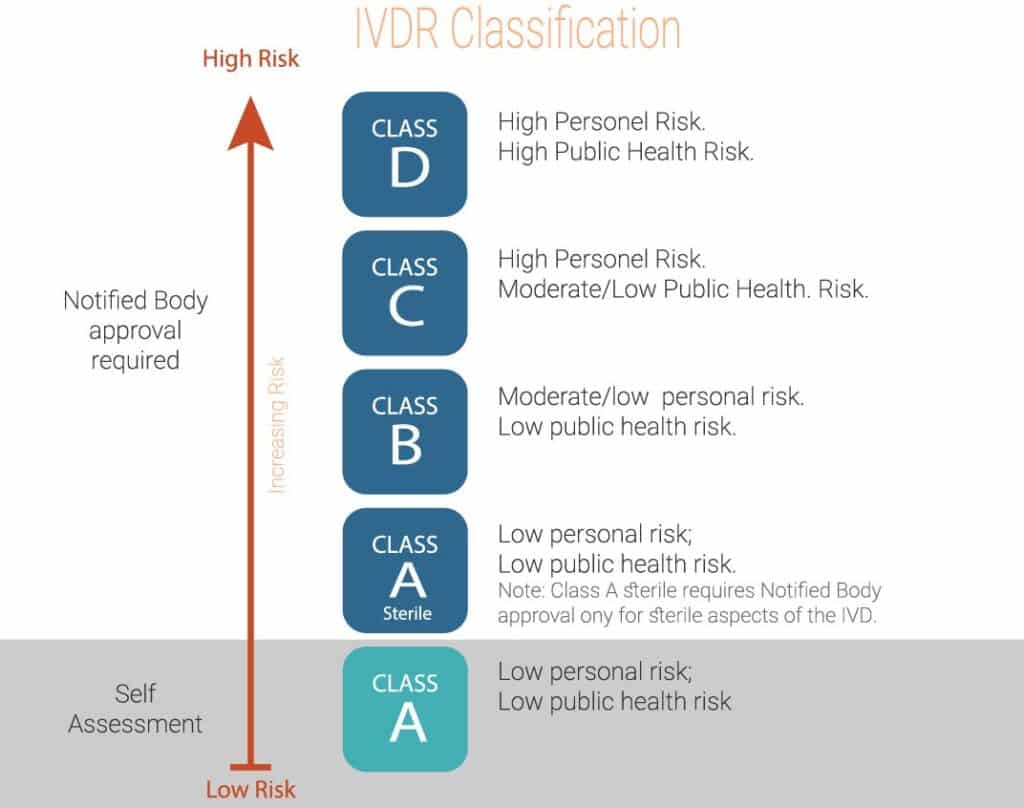
RS NESS is a highly experienced service provider in the Medical industry.
RS-Ness can assist you with an IVDR gap assessment to help you create an appropriate project plan, and support the transition throughout the Regulatory, Clinical, QA, Validation, and Engineering processes.
We differentiate ourselves by being quality-oriented and by our technical expertise that comes with a hands-on experience and approach, ensuring that our clients receive the most effective and professional service.
Our clients range from small Start-Ups to international companies.
Butterfly effect due to a small part exchange in a Medical device company
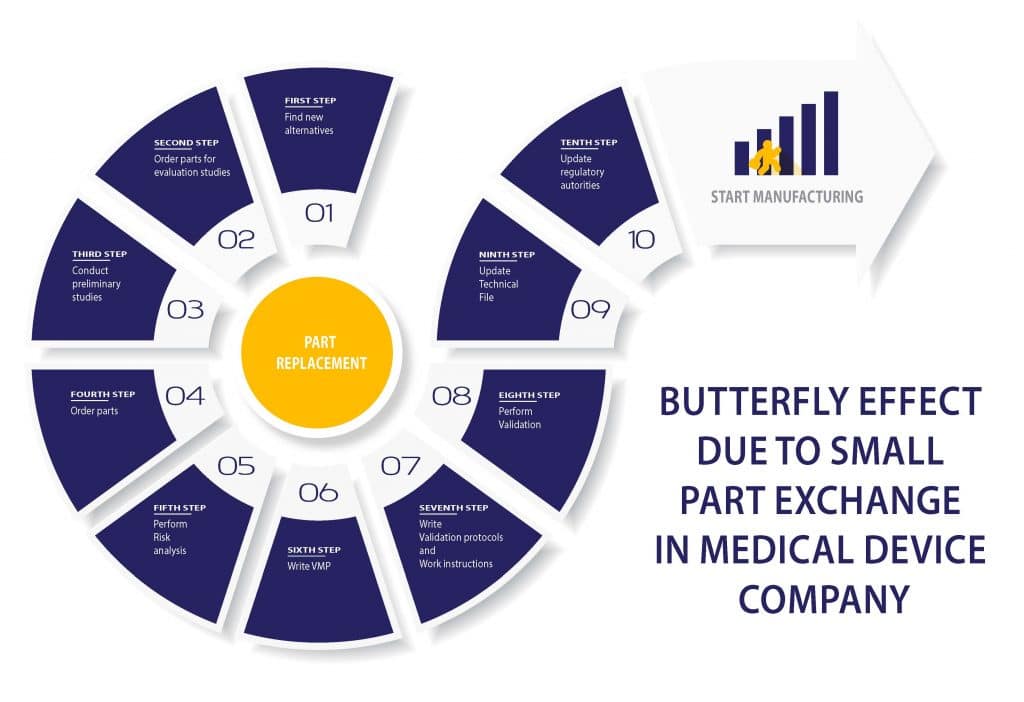
RS NESS is a service provider and consulting company in the Life science industry for those who are unfamiliar with us.
RS NESS provides a variety of services such as project management, quality assurance, regulatory affairs, clinical affairs, validation and engineering services.
As an employee at RS NESS, in addition to our academic knowledge and practical training, we have been gifted by the ability to learn and adapt quickly, to combine our expertise with flexibility in order to provide the most professional service to our customers.
Cleanroom Design
RS-NESS works with various companies to construct production facilities and provide a comprehensive solution for these complex projects. We accompany the customer from the initial stages – planning in principle and obtaining approval of the facility and perform validations for equipment / systems /processes while accompanying and supervising the entire project on behalf of the customer.
If you have any questions, or if you need professional support, please contact us.
Clean Room Construction Project
RS-NESS works with various companies to construct production facilities and provide a comprehensive solution for these complex projects. We accompany the customer from the initial stages – planning in principle and obtaining approval of the facility and perform validations for equipment / systems /processes while accompanying and supervising the entire project on behalf of the customer.
If you have any questions, or if you need professional support, please contact us.
Preparing your QMS for the EU MDR

RS Ness supports Life Science companies in QA, Regulatory Affairs, Clinical Affairs, Engineering, Validation and Project Management.
Contact us to support your MDR Transition.
How to establish DHR

RS NESS supports the Life Science in such field as: Quality Assurance (QA), Regulatory Affairs (RA), Clinical Affairs (CA), Engineering, Validation and Project Management (PM). We provide service to the companies at different lifecycle stages incorporating end-to-end project activities while adhering to the regulatory requirements. Knowledge, professionalism, and dedication lead our highly qualified team to your success.
If you have any questions, or if you need professional support, please contact us.
Medical Device Roadmap from Idea to Market
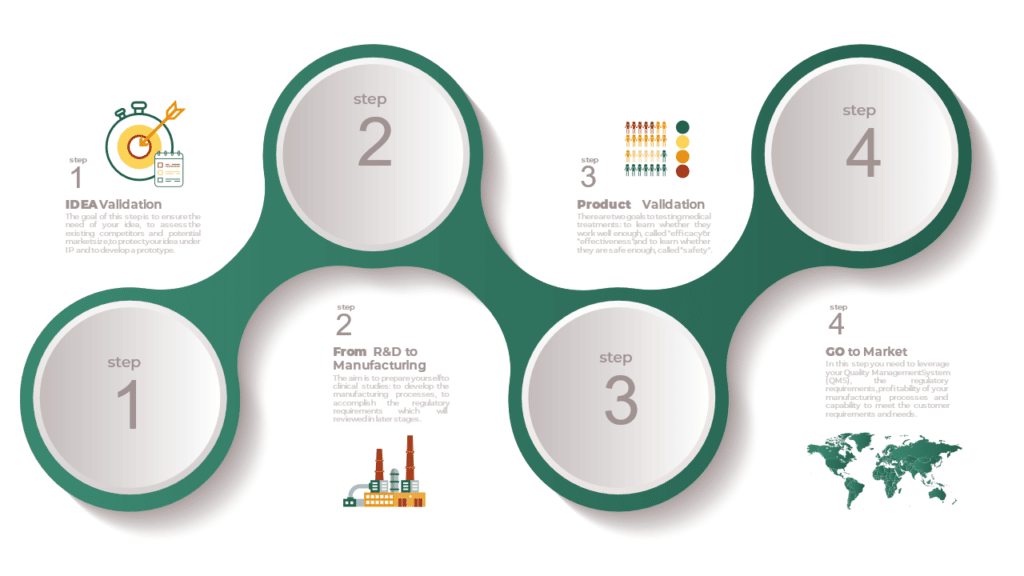
Commercializing medical technology is challenging, often requiring 10 – 20 years just to bring new ideas to market. There is an essential need for better understanding of the overall process to the market. Challenges arising from time delays, misalignment with regulatory requirements and resource constraints potentially amount to substantial losses in value, for both companies […]
Your Packaging Validation Guideline

RS NESS is a highly experienced service provider in the Medical industry. RS Ness can assist you with Packaging and Shipping validation, to help you create an appropriate project plan, and support the transition throughout the Regulatory, Clinical, QA, Validation, and Engineering processes.
We differentiate ourselves by being quality-oriented and by our technical expertise that comes with a hands-on experience and approach, ensuring that our clients receive the most effective and professional service.
Our clients range from small Start-Ups to international companies.
If you have any questions, or if you need professional support, please contact us.
The Nanosono journey- from idea to ISO13485 Certification
RS NESS supports Medical device companies in rapid scale-up and meeting GMP compliance requirements; proving the experience you need when you need it.
If you are planning to get ISO13485 certification, please contact us for support.
Validation of Medical Device manufacturing line for getting the European CE mark certification.

RS NESS is a highly experienced service provider in the Medical industry.
RS-Ness can assist you with a gap assessment to help you create an appropriate project plan, and support the transition throughout the Regulatory, Clinical, QA, Validation, and Engineering processes.
We differentiate ourselves by being quality-oriented and by our technical expertise that comes with a hands-on experience and approach, ensuring that our clients receive the most effective and professional service.
Our clients range from small Start-Ups to international companies.
If you have any questions, or if you need professional support, please contact us.
Supplier evaluation and approval in 5 steps.

RS-NESS supports Life Science companies in rapid scale-up and meeting GMP compliance requirements; proving the experience you need when you need it.
If you are planning a Supplier Evaluation and Approval process, contact us to discuss how we can support your goals.
Preparing your Quality Management System for the MDR: QA Best Practices

RS-Ness supports Life Science companies in QA, Regulatory Affairs, Clinical affairs, Validation and Project Management.
We will assist you with an MDR gap assessment to help you create an appropriate project plan, and support the transition throughout the Regulatory, Clinical, QA, Validation, and Engineering processes.
Our clients range from small Start-Ups to international companies.
If you have any questions, or if you need professional support, please contact us.
If you have 2 months to establish an EU-GMP compliant production line for Medical Cannabis, what should you do?

RS NESS supports Life Science companies in rapid scale-up and meeting GMP compliance requirements; proving the experience you need, when you need it.
If you are planning your production validation, contact us to discuss how we can support your goals
Process Validation: Pharma vs. Medical Device

RS-NESS provides an umbrella of services to the Life Science industry in the areas of validation, quality assurance, regulatory affairs, clinical affairs, project management, and engineering. This approach enables a “one-stop-shop”, incorporating end-to-end project activities while adhering to the regulatory requirements. Knowledge, professionalism, and dedication lead our highly qualified team to your success.
MDSAP In 60 Sec

For those who are not familiar with the Medical Device Single Audit Program (MDSAP), it is a harmonized approach for auditing and monitoring medical device manufacturers quality management system on an international scale. MDSAP program allows a single regulatory audit of a medical device manufacturer’s quality management system to satisfy the needs of participating regulatory […]












